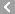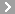CAS No.:59-52-9
Name: 2,3-Dimercapto-1-propanol
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TDLo | intramuscular | 3mg/kg (3mg/kg) | BLOOD: HEMORRHAGE SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" |
Science. Vol. 102, Pg. 601, 1945. |
| mouse | LD50 | intramuscular | 113mg/kg (113mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 223, Pg. 408, 1954. | |
| mouse | LD50 | intraperitoneal | 25mg/kg (25mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | 56mg/kg (56mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#04985, | |
| mouse | LD50 | oral | 217mg/kg (217mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 21, Pg. 364, 1948. | |
| mouse | LD50 | unreported | 99mg/kg (99mg/kg) | Fukuoka Igaku Zasshi. Vol. 49, Pg. 2779, 1958. | |
| rabbit | LD50 | intramuscular | 50mg/kg (50mg/kg) | Biochemical Journal. Vol. 41, Pg. 325, 1947. | |
| rabbit | LD50 | intravenous | 50mg/kg (50mg/kg) | Biochemical Journal. Vol. 41, Pg. 325, 1947. | |
| rabbit | LD50 | parenteral | 40mg/kg (40mg/kg) | VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION VASCULAR: REGIONAL OR GENERAL ARTERIOLAR CONSTRICTION |
Annales Pharmaceutiques Francaises. Vol. 5, Pg. 172, 1947. |
| rabbit | LD50 | unreported | 80mg/kg (80mg/kg) | Fukuoka Igaku Zasshi. Vol. 49, Pg. 2779, 1958. | |
| rat | LD50 | intramuscular | 87mg/kg (87mg/kg) | Toxicology and Applied Pharmacology. Vol. 36, Pg. 297, 1976. | |
| rat | LD50 | intraperitoneal | 105mg/kg (105mg/kg) | Annales Pharmaceutiques Francaises. Vol. 5, Pg. 172, 1947. | |
| rat | LD50 | subcutaneous | 2gm/kg (2000mg/kg) | Annales Pharmaceutiques Francaises. Vol. 5, Pg. 172, 1947. | |
| rat | LD50 | unreported | 113mg/kg (113mg/kg) | Fukuoka Igaku Zasshi. Vol. 49, Pg. 2779, 1958. |
Consensus Reports:
EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
SAFETY PROFILE:
Poison via ingestion, intramuscular, parenteral, intraperitoneal, and intravenous routes. Experimental teratogenic effects. Human systemic effects by intramuscular route: hemorrhage and dermatitis. Human blood and systemic skin effects by intramuscular route. It causes redness and swelling when applied locally to the skin, but does not produce blisters or ulcers. Intensely irritating to eyes and mucous membranes. Systemic symptoms are caused by injection. When heated to decomposition, it emits toxic fumes of SOx. Used as an antidote to arsenic, gold, and mercury poisoning.
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 22-36/37/38-36/38
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
R36/38:Irritating to eyes and skin.
Safety Statements: 26-36-24/25-23
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
S23:Do not breathe vapour.
RIDADR: UN 2810 6.1/PG 3
WGK Germany: 3
F: 8-9-13-23
HazardClass: 6.1
PackingGroup: III
RTECS: UB2625000
Standards and Recommendations:
Analytical Methods:
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione