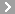CAS No.:55-98-1
Name: Busulfan
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 56200ug/kg (56.2mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| dog | LDLo | intravenous | 8mg/kg (8mg/kg) | BLOOD: LEUKOPENIA BLOOD: AGRANULOCYTOSIS BLOOD: OTHER CHANGES |
Cancer Chemotherapy Reports, Part 2. Vol. 2, Pg. 203, 1965. |
| man | TDLo | oral | 8mg/kg/2D-I (8mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Lancet. Vol. 2, Pg. 1463, 1984. |
| monkey | LDLo | intravenous | 8mg/kg (8mg/kg) | BLOOD: LEUKOPENIA BLOOD: OTHER CHANGES BLOOD: AGRANULOCYTOSIS |
Cancer Chemotherapy Reports, Part 2. Vol. 2, Pg. 203, 1965. |
| mouse | LD50 | intraperitoneal | 86mg/kg (86mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" |
Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | oral | 110mg/kg (110mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): HAIR: OTHER |
Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | subcutaneous | 63mg/kg (63mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" |
Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | unreported | 46mg/kg (46mg/kg) | British Journal of Cancer. Vol. 6, Pg. 160, 1952. | |
| rat | LD50 | intraperitoneal | 18mg/kg (18mg/kg) | GASTROINTESTINAL: OTHER CHANGES BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE |
Biochemical Pharmacology. Vol. 1, Pg. 39, 1958. |
| rat | LD50 | intravenous | 1800ug/kg (1.8mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 20, Pg. 1467, 1970. | |
| rat | LD50 | subcutaneous | 22mg/kg (22mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" |
Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| rat | LD50 | unreported | 10mg/kg (10mg/kg) | Chemical Abstracts. Vol. 55, Pg. 10679a, 1961. | |
| rat | LDLo | oral | 15mg/kg (15mg/kg) | Khimiya i Meditsina. Vol. 13, Pg. 34, 1960. | |
| women | TDLo | oral | 80mg/kg/8Y (80mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS |
JAMA, Journal of the American Medical Association. Vol. 238, Pg. 1951, 1977. |
Consensus Reports:
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 137.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 4 (1974),p. 247.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 4 (1974),p. 247.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program.
SAFETY PROFILE:
Confirmed carcinogen producing leukemia, kidney, and uterine tumors. Experimental neoplastigenic and tumorigenic data. Poison by ingestion, subcutaneous, intraperitoneal, intravenous, and possibly other routes. Ingestion by pregnant women can cause cancer of the reproductive system of the fetus including the uterus. Human teratogenic effects by ingestion and possibly other routes include developmental abnormalities of the eye, ear, craniofacial area including the nose and tongue, gastrointestinal system, endocrine system, urogenital system, and other unspecified areas. Other human reproductive effects by ingestion and possibly other routes include: impotence, changes in the uterus, cervix, and vagina, and menstrual-cycle disorders. Experimental reproductive effects. Human systemic effects by ingestion: general arteriolar or venous dilation of the eye, changes in structure or function of salivary glands. When heated to decomposition it emits toxic fumes of SOx. See also Hazard SULFONATES.
Codes: T+ ,T
,T
Risk Statements:
23: Toxic by inhalation
24: Toxic in contact with skin
25: Toxic if swallowed
26: Very Toxic by inhalation
27: Very Toxic in contact with skin
28: Very Toxic if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
45: May cause cancer
46: May cause heritable genetic damage
63: Possible risk of harm to the unborn child
Safety Statements:
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
63: In case of accident by inhalation, remove casualty to fresh air and keep at rest
RIDADR: UN 2811 6.1/PG 1
WGK Germany: 3
RTECS: EK1750000
HazardClass: 6.1(b)
PackingGroup: III
Standards and Recommendations:
Analytical Methods:
Related Searches

Related Suppliers
- Ganyu Unitechemical Co., Ltd.
- Ash Stevens, Inc.
- Sichuan Credit Chemwerth Pharmaceutical Co., Ltd
- Shilpa Medicare Ltd
- Pfaltz & Bauer, Inc.
- Jinan Great Chemical Co.,Ltd.
- Baoji Guokang Bio-Technology Co.,Ltd
- FRESENIUS-KABI ONCOLOGY LTD
- Chemieliva Pharmaceutical Co., Ltd.
- Hangzhou Dawn Ray Pharmaceutical Co.,Ltd
- Xi'an Costrong Pharmaceutical Co., Ltd.
- ChemWerth
- Interchem Corporation
- Taizhou Crene Biotechnology co.ltd
- Shaanxi King Stone Enterprise Company Limited
- ChemWerth Inc.
- shanxi top pharm chem
- SHAANXI TOP PHARM CHEMICAL CO.LTD
- Hubei Hengluyuang Technology Co.,Ltd
- EXCELLA GmbH
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione