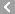CAS No.:83905-01-5
Name: Azithromycin
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | TDLo | oral | 21mg/kg/3D-I (21mg/kg) | LIVER: "JAUNDICE, CHOLESTATIC" KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION BLOOD: "CHANGES IN SERUM COMPOSITION (E.G., TP, BILIRUBIN, CHOLESTEROL)" |
American Journal of Medicine. Vol. 102, Pg. 217, 1997. |
| man | TDLo | oral | 32mg/kg/9D-I (32mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: CHANGES IN BOTH TUBULES AND GLOMERULI KIDNEY, URETER, AND BLADDER: HEMATURIA |
Annals of Internal Medicine. Vol. 119, Pg. 636, 1993. |
| mouse | LD50 | oral | 3gm/kg (3000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION GASTROINTESTINAL: NAUSEA OR VOMITING |
Oyo Yakuri. Pharmacometrics. Vol. 51, Pg. 53, 1996. |
| mouse | LDLo | intraperitoneal | 400mg/kg (400mg/kg) | BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION GASTROINTESTINAL: NAUSEA OR VOMITING |
Oyo Yakuri. Pharmacometrics. Vol. 51, Pg. 53, 1996. |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION GASTROINTESTINAL: NAUSEA OR VOMITING |
Oyo Yakuri. Pharmacometrics. Vol. 51, Pg. 53, 1996. |
| rat | LDLo | intraperitoneal | 900mg/kg (900mg/kg) | BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION GASTROINTESTINAL: NAUSEA OR VOMITING |
Oyo Yakuri. Pharmacometrics. Vol. 51, Pg. 53, 1996. |
Consensus Reports:
SAFETY PROFILE:
Hazard Codes of Azithromycin (CAS NO.83905-01-5):  Xi
Xi
RTECS: RN6960000
The Most common side effects are gastrointestinal; diarrhea , nausea , abdominal pain and vomiting.Serious allergic reactions, nervousness, dermatologic reactions, and fatalities have been reported extremely rare.As with all antimicrobial agents, pseudomembranous colitis can occur during and up to several weeks after azithromycin therapy. Azithromycin may interfere with the effectiveness of birth control pills; other forms of contraception may be required during the treatment period.
Standards and Recommendations:
Analytical Methods:
Related Searches

Related Suppliers
- Wuhan Fortuna Chemical Co.,Ltd
- LIDE PHARMACEUTICALS LIMITED
- Afine Chemicals Limited
- Simagchem Corporation
- Antimex Chemical Limied
- Josun International Limited
- Jinan Finer Chemical Co., Ltd
- Hangzhou JINLAN Pharm-Drugs Technology Co., Ltd
- Kono Chem Co.,Ltd
- BOC Sciences
- Premier Drug House
- X.T.Y Environ-Tech.Co.,Ltd
- Sanjeevani Biotech Pvt. Ltd.
- Vaishali Pharma Pvt. Ltd.
- Wuhan Benjamin Pharmaceutical Chemical Co.,Ltd
- Beijing Boyakaihua Company
- TODY BROTHERS PVT.LTD.
- GORANG INTERNATIONAL
- GORANG INTERNATIONAL
- J. B. CHEMICALS & PHARMACEUTICALS LIMITED
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione