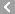CAS No.:59-05-2
Name: Methotrexate
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | intravenous | 100mg/kg/4H (100mg/kg) | BLOOD: THROMBOCYTOPENIA BLOOD: OTHER CHANGES |
Cancer Vol. 33, Pg. 1151, 1974. |
| child | TDLo | oral | 2mg/kg/12D (2mg/kg) | LUNGS, THORAX, OR RESPIRATION: COUGH LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
JAMA, Journal of the American Medical Association. Vol. 209, Pg. 1861, 1969. |
| human | TDLo | intramuscular | 35mg/kg/28W (35mg/kg) | VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: CYANOSIS |
British Medical Journal. Vol. 2, Pg. 156, 1970. |
| human | TDLo | intramuscular | 200mg/kg/5Y (200mg/kg) | LIVER: "HEPATITIS, FIBROUS (CIRRHOSIS, POST-NECROTIC SCARRING)" | Archives of Dermatology. Vol. 100, Pg. 531, 1969. |
| human | TDLo | intravenous | 4650ug/kg/4W- (4.65mg/kg) | LIVER: FATTY LIVER DEGERATION LIVER: LIVER FUNCTION TESTS IMPAIRED |
Proceedings of the American Association for Cancer Research. Vol. 5, Pg. 26, 1964. |
| human | TDLo | intravenous | 7143ug/kg (7.143mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BLOOD: CHANGES IN PLATELET COUNT BLOOD: CHANGES IN LEUCOCYTE (WBC) COUNT |
Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 673, 1992. |
| human | TDLo | oral | 43mg/kg/5Y (43mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED LIVER: OTHER CHANGES |
Archives of Dermatology. Vol. 100, Pg. 523, 1969. |
| man | TDLo | intramuscular | 214ug/kg/12D- (0.214mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Clinical and Experimental Rheumatology. Vol. 14, Pg. 450, 1996. |
| man | TDLo | intravenous | 740mg/kg (740mg/kg) | GASTROINTESTINAL: OTHER CHANGES | Archives of Internal Medicine. Vol. 136, Pg. 1321, 1976. |
| man | TDLo | oral | 643ug/kg/6W-I (0.643mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Rheumatology. Vol. 14, Pg. 74, 1987. |
| man | TDLo | oral | 4286ug/kg/2.7 (4.286mg/kg) | LUNGS, THORAX, OR RESPIRATION: "FIBROSIS, FOCAL (PNEUMOCONIOSIS)" LUNGS, THORAX, OR RESPIRATION: RESPIRATORY OBSTRUCTION BLOOD: APLASTIC ANEMIA |
Clinical Rheumatology. Vol. 12, Pg. 535, 1993. |
| mouse | LD50 | intraperitoneal | 50mg/kg (50mg/kg) | Anatomical Record. Vol. 178, Pg. 465, 1974. | |
| mouse | LD50 | intravenous | 65mg/kg (65mg/kg) | Drugs in Japan Vol. 6, Pg. 841, 1982. | |
| mouse | LD50 | oral | 146mg/kg (146mg/kg) | Drugs in Japan Vol. 6, Pg. 841, 1982. | |
| mouse | LD50 | subcutaneous | 250mg/kg (250mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | unreported | 69mg/kg (69mg/kg) | Cancer Research. Vol. 46, Pg. 2703, 1986. Link to PubMed |
|
| rat | LD50 | intraperitoneal | 6mg/kg (6mg/kg) | Drugs in Japan Vol. 6, Pg. 841, 1982. | |
| rat | LD50 | intravenous | 14mg/kg (14mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 20, Pg. 1467, 1970. Link to PubMed |
|
| rat | LD50 | oral | 135mg/kg (135mg/kg) | Drugs in Japan Vol. 6, Pg. 841, 1982. | |
| rat | LD50 | subcutaneous | 58mg/kg (58mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 93, 1992. | |
| rat | LD50 | unreported | 133ug/kg (0.133mg/kg) | United States Patent Document. Vol. #4746662, | |
| women | LDLo | intraspinal | 36mg/kg/15D (36mg/kg) | SPINAL CORD: OTHER DEGENERATIVE CHANGES GASTROINTESTINAL: NAUSEA OR VOMITING |
New England Journal of Medicine. Vol. 289, Pg. 770, 1973. |
| women | TDLo | oral | 800ug/kg/4D-I (0.8mg/kg) | BLOOD: LEUKOPENIA BLOOD: THROMBOCYTOPENIA BLOOD: OXIDANT RELATED (GPD DEFICIENT) ANEMIA |
Medical Journal of Australia. Vol. 155, Pg. 493, 1991. |
| women | TDLo | oral | 2mg/kg/17W-I (2mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BLOOD: LEUKOPENIA |
Journal of Rheumatology. Vol. 14, Pg. 74, 1987. |
| women | TDLo | parenteral | 2600ug/kg (2.6mg/kg) | BRAIN AND COVERINGS: CHANGES IN CEREBRAL SPINAL FLUID LUNGS, THORAX, OR RESPIRATION: "FIBROSIS, FOCAL (PNEUMOCONIOSIS)" LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Cancer Vol. 38, Pg. 1529, 1976. |
| women | TDLo | unreported | 11400ug/kg/44 (11.4mg/kg) | LUNGS, THORAX, OR RESPIRATION: "FIBROSIS, FOCAL (PNEUMOCONIOSIS)" | Clinical and Experimental Rheumatology. Vol. 15, Pg. 583, 1997. |
| women | TDLo | unreported | 150mg/kg (150mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | Cancer Vol. 48, Pg. 2158, 1981. |
Consensus Reports:
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 241.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 26 ,1981,p. 267.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 26 ,1981,p. 267.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Studies (ipr); No Evidence: mouse, rat CANCAR Cancer. 40 (1977),1935. . Reported in EPA TSCA Inventory.
SAFETY PROFILE:
A human poison by intraspinal route. Poison experimentally by ingestion, intravenous, subcutaneous, and intraperitoneal routes. Human teratogenic effects by ingestion: developmental abnormalities of the craniofacial area and the musculoskeletal system. Human systemic effects by multiple routes: thrombocytopenia (decrease in the number of blood platelets), bone marrow changes, other blood changes, cerebral spinal fluid effects, eye effects, blood pressure lowering, cough, dyspnea, fibrosis (pneumoconiosis), cyanosis, gastrointestinal effects, fatty liver degeneration, hepatitis, impairment of liver function tests, other liver changes, fever, effects on inflammation or mediation of inflammation, leukopenia. Human mutation data reported. Experimental reproductive effects. A human eye irritant. Questionable human carcinogen producing leukemia, Hodgkin's disease, and skin tumors. An FDA proprietary drug. A chemotherapeutic agent. When heated to decomposition it emits toxic fumes including NOx.
Hazard Codes:  T
T
Risk Statements: 61-25-36/38-46
R61: May cause harm to the unborn child.
R25: Toxic if swallowed.
R36/38: Irritating to eyes and skin.
R46: May cause heritable genetic damage.
Safety Statements: 53-26-36/37-45-36/37/39
S53: Avoid exposure - obtain special instructions before use.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37: Wear suitable protective clothing and gloves.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
RTECS: MA1225000
F: 3-8-10
HazardClass: 6.1(b)
PackingGroup: III
Standards and Recommendations:
Analytical Methods:
Related Searches

Related Suppliers
- Afine Chemicals Limited
- Dayang Chem (Hangzhou) Co.,Ltd.
- Jai Radhe Sales
- HANWAYS CHEMPHARM CO.,LIMITED
- Hangzhou Ocean Chemical Co., Ltd.
- Xiamen Hisunny Chemical Co.,Ltd
- Hangzhou JINLAN Pharm-Drugs Technology Co., Ltd
- Cygnus HealthCare Specialities P Ltd
- ATHENE CHEMICALS PVT. LTD.
- Vesta Pharmachem Private Limited
- Welfull Group Co., Ltd.
- Lianyungang Guiyuan(guike) Chempharm Co., Ltd.
- Shanghai agrotree chemical co.,ltd.
- SIHONG BONA INDUSTRY AND TRADE CO.,LTD.
- Shaanxi King Stone Enterprise Company Limited
- Showa best glove
- SHAANXI TOP PHARM CHEMICAL CO.LTD
- EXCELLA GmbH
- Hockley International Ltd
- Amronco
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione